Advanced pulmonary and cancer drug “Ofenib” manufactured locally within the Iran International Innovation District (IIID).
Iran achieves domestic production of Ofenib, reducing import dependence and enhancing pharmaceutical sovereignty.
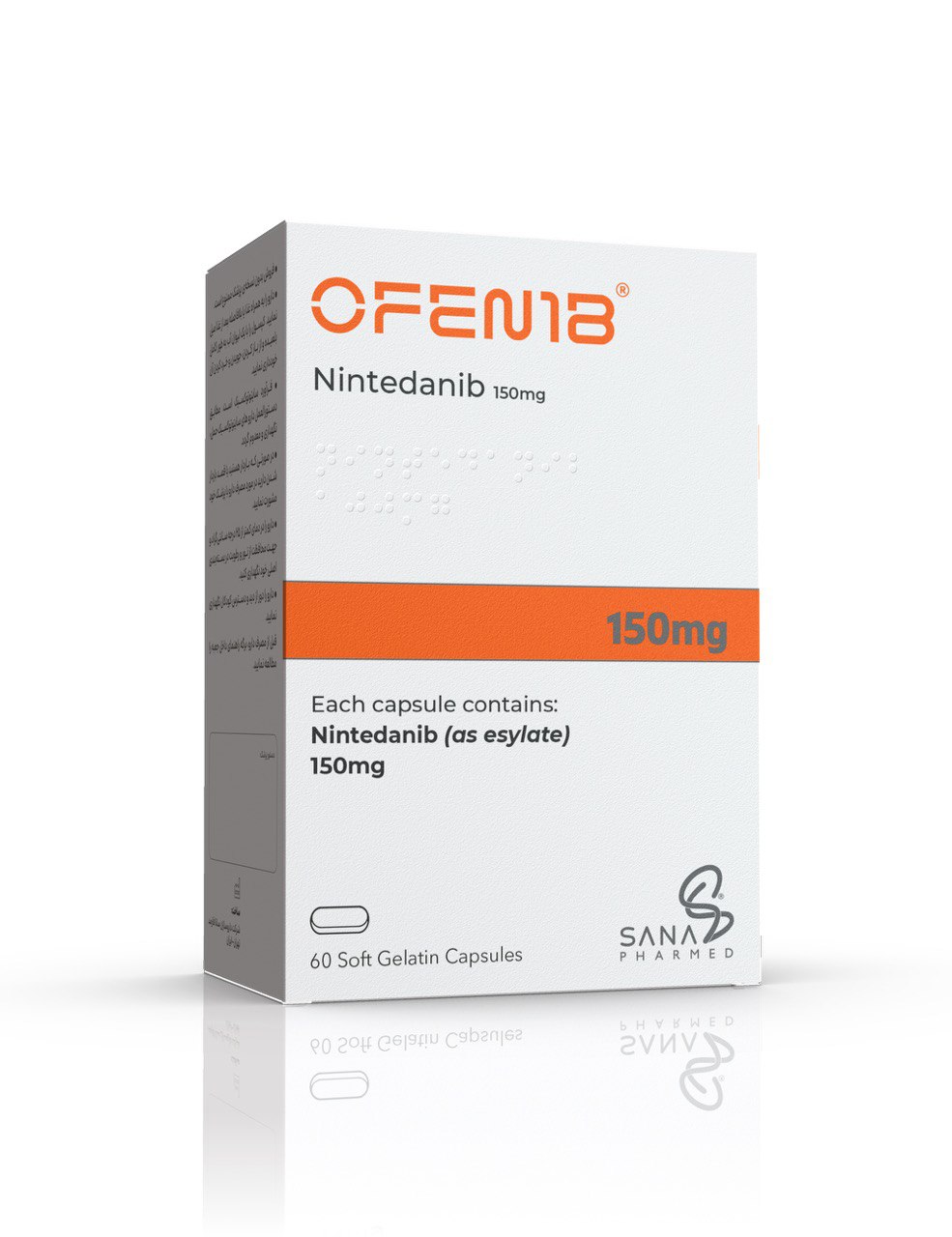
Iranian biotech firm Sana Pharmed, a member of the Azadi Innovation Factory under IIID, has successfully indigenized the advanced drug nintedanib (marketed as Ofenib), previously available only in limited imported quantities. The domestically produced formulation not only ensures reliable access for patients with severe lung and cancer conditions but also positions Iran for export opportunities across regional markets.
Sana Pharmed unveiled the locally manufactured Ofenib during the 23rd annual meeting of IIID. Nintedanib is one of the few globally approved treatments for idiopathic pulmonary fibrosis, progressive fibrotic lung diseases, scleroderma-related lung disease, and adenocarcinoma-type lung cancer.
CEO Saman Pourzia stated that Ofenib works by inhibiting key fibrosis pathways, slowing the stiffening and scarring of lung tissue. The drug offers proven clinical efficacy, ease of administration, and improved tolerability for a wide range of patients.
Domestic manufacturing has significantly lowered treatment costs. While patients previously paid over USD 1,000 per month for imported doses, the current cost has dropped to approximately 20% of the imported price. The project required more than IRR 150 billion in research and development investment.
Pourzia emphasized that prior to local production, nintedanib reached patients through single-prescription channels or smuggling, leading to severe access and cost challenges. Local manufacturing eliminates these barriers, saves foreign currency, strengthens national drug security, and creates export potential to neighboring countries.
In addition to Ofenib, Sana Pharmed’s portfolio includes Sanarelbin (anti-cancer), Acnotherap (for severe acne), and Saditrol (vitamin D metabolites), reinforcing its position in high-tech speciality pharmaceuticals. The company operates from Azadi Innovation Factory’s branch within IIID, focusing on the development of niche drugs for domestic and regional healthcare needs.
Quick Access
Address: Pardis Technology Park, 20th km of Damavand Road (Main Stresst), Tehran I.R. Iran.
Postal Code: 1657163871

Tel: 76250250 _ 021

Fax: 76250100 _ 021
E-mail: info@techpark.ir

website:iiid.tech